Looking for an
outlet for excess Chlorine?
Need to dispose of Scrap Iron?
Iron(III) chloride, FeCl3, molecular
weight 162.2, also called ferric chloride, is an almost black crystalline solid.
It is very soluble in water and a strong oxidising agent.
Iron(III) chloride is mainly used as flocculating and precipitating agent in
the treatment of both drinking water and waste water. When small quantities
of iron(III) chloride are added to the raw water, iron(III) hydroxide precipitates
and adsorbs finely divided solids and colloids. Smaller amounts of iron(III)
chloride are used as chlorinating agent and catalyst in the chemical industry
and for etching in the electronic and printing industries. The
annual production exceeds 600'000 t. The major commercial form is the solution
with 40 % FeCl3, whereas the market for anhydrous iron(III) chloride is very
limited. This document provides information on the manufacture of iron(III)
chloride solution from scarp iron and chlorine.
Iron(III) chloride is a strong oxidising
agent and readily dissolves metallic iron to iron(II) chloride: 2 FeCl3 + Fe
---> 3 FeCl2
A number of other reactions such as the formation of hydrogen, hydrogen sulfide
and phosphine may occur simultaneously and may lead to a reduced iron yield,
safety problems and air pollution. Krebs Swiss have thoroughly investigated
the complex mechanisms which take place in the iron dissolvers and have succeeded
to optimise the operating parameters in such a way that the hydrogen formation
is nominal and the smell negligible.
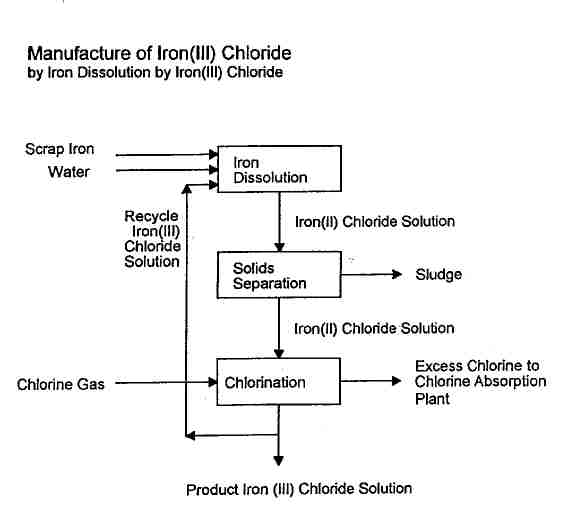
The application of Krebs Swiss' comprehensive and sophisticated know-how results in a simplified and more economic plant for the production of iron(III) chloride solution from scrap iron and chlorine as outlined in the flowsheet: The iron(II) chloride solution resulting from iron dissolution is clarified and oxidized to iron(III) chloride. Two third of the iron(III) chloride solution are recycled back to the iron dissolution and one third is branched off as product.
The scrap iron is added at intervals to a dissolving tank through which iron(III)
chloride solution is continuously circulated and converted to iron(II) chloride
solution.
Suspended solids contained in the iron(II) chloride solution which overflows
from the iron dissolving tank are separated in a clarifier. The underflow of
the clarifier is mixed with hydrochloric acid for maximum recovery of the iron
oxides and hydroxides. The residual solids are separated by filtration and the
filtrate is added again to the clarifier overflow.
For chlorination to Iron(III) chloride, the clarified iron(II) chloride solution
is fed to a packed column with recirculation pump and cooler for the recirculated
solution. Most of the chlorine fed to the column is absorbed and reduced to
chloride while oxidising iron(II) to iron(III): 3 FeCl2 + 1 1/2 Cl2 --->
3 FeCl3
The excess chlorine gas is directed
to an existing chlorine absorption plant. Alternatively, additional absorption
columns are installed if the tail gas is to be vented directly over roof.
One third of the resulting iron(III) chloride solution is branched off as product
and the other two third are recycled back to the iron dissolving tank for production
of more iron(II) chloride.
The process is easy to practise and very suitable as a low investment consumer
of surplus chlorine. The raw materials are likewise cheap and easily available
so that the manufacture of iron (III) chloride frequently is an attractive supplement
for chloralkali producers.

The most suitable form of scrap iron are turnings from steel machining and cuttings from can manufacturing or other punching operations. It should be free from oil and grease to prevent the formation of chlororganic compounds. SInce not only the iron but also any other metals contained in the scrap are dissolved, the purity of scrap iron affects the purity of the product solution.
Also with respect to the chlorine
source, the process is flexible. The use of controlled quantities of evaporated
liquid chlorine results in the lowest investment. With some additional installations,
other forms such as cell gas chlorine with hydrogen, or dilute chlorine gas
or uncontrolled quantities of chlorine can also be utilised.
The only raw material additionally required in significant quantities is water.
The process described above yields
a aqueous solution with minimum 40 % FeCl3 and max. 0.2 % FeCl2. The solution
meets the requirements of DIN standard 19'602. The amount of impurities contained
in the solution depends on the amount of impurities contained in the scrap iron.
The main operational requirements
for the manufacture of 1'000 kg FeCl3 by the process described above are about
as follows:
Chlorine: 660 to 730 kg Cl2
Scrap Iron: 360 kg Fe
Hydrochloric acid: 25 kg HCl
Process water: 2 m3
Electric power: 35 kWh
Cooling water: 40 m3
An iron(III) chloride plant can easily
be operated by the crew which operated the other sections of a chloralkali plant,
so that no extra shift personnel is required. Refilling of the scrap iron dissolving
tank and other work intermittent work is usually performed by one operator in
the day shift.
Likewise, by analytical work can well be performed by the team performing such
work for the overall chloralkali plant.